In most cases, a partial meniscectomy is performed after a meniscus injury. In the years that follow, a post-meniscectomy syndrome develops with secondary arthrosis. This situation then leads to a corresponding prosthetic treatment. arious techniques for cartilage regeneration are described in the literature. These include autologous cartilage transplantation and cartilage induction with a cell-free matrix...
Autor:
Olaf Thorsten Beck M.D.
Specialist in orthopaedics, trauma surgery, surgery and sports medicine
Weisser Str. 22, D-50996 Köln/Colonia/Cologne
info@arthro-prax.de
Introduction:
In most cases, a partial meniscectomy is performed after a meniscus injury. In the years that follow, a post-meniscectomy syndrome develops with secondary arthrosis. This situation then leads to a corresponding prosthetic treatment.
Various techniques for cartilage regeneration are described in the literature. These include autologous cartilage transplantation and cartilage induction with a cell-free matrix, collagen type 1, as well as various meniscus replacement techniques. In the past, the CMI implant made from bovine Achilles tendons was the market leader. However, production was discontinued in 2021. From this point onwards, only the Actifit implant was available as a partial replacement. However, unlike the CMI, this was not a biological implant but was made of polyurethane. Various studies have shown that the outcome was inferior to that of the CMI. A new biological implant has been available in Germany since November 2022 and can currently be used by 4 doctors in Germany. This is a cadaveric cancellous bone block that is demineralised and free of DNA. The implant is supplied sterile and dry. Studies on a combination of cartilage regeneration in connection with meniscus implantation are currently not available.
Description of the technique:
The patient must have a maximum malalignment of varus and valgus of 5°. Other contraindications include joint stiffness, chronic joint inflammation such as rheumatism, psoriatic arthritis and arthritis urica. Allergies to bovine proteins are also a contraindication.
Sufficient patient compliance is also important, as the success of the operation depends to a large extent on adherence to the postoperative regimen.
So far, I have treated patients up to a maximum age of 65 years with this procedure. However, concomitant illnesses in particular must be taken into account here. Patients who have to take cortisone or anti-rheumatic drugs regularly have been rejected by me.
It is also important to make the correct diagnosis using imaging, which is only possible with the help of an MRI. If there are bone changes in the sense of bone oedema, appropriate relief should be provided using an orthosis and focussed shock wave therapy. The frequency of application depends on the size of the area. On average, I carry out 4 applications at intervals of 1 week. A control MRI is carried out 6 weeks after the last shock wave therapy.
The size of the cartilage defect and the edge of the meniscus must also be determined. The geometry of the cartilage defect is important. Cartilage induction is possible if the defect is up to 1.5cm wide. The length then plays no further role. Based on our postoperative scheme, the treatment of kissing lesions is also possible.
The residual meniscus should have a rim of 3mm and intact fixation of the anterior and posterior horn.
If this information cannot be determined by MRI, I perform a nanoscopy under local anaesthetic for planning purposes.
During the arthroscopy, the first step is to prepare the cartilage using a ring curette. The aberat is removed using a shaver. The meniscus implant (Spongioflex) is placed in saline for approx. 15 minutes until it is soft. Using meniscus grasping forceps, the implant is inserted into the joint through a mini-open incision and fixed in place with all-inside sutures. The procedure then switches from water arthroscopy to gas arthroscopy. CO2 is used here. The sufflator is connected via a gas hose that can be connected to the arthroscopy trocar.
After the cartilage defect sites have dried, they are covered with a collagen gel.
Own Results:
o date, no published follow-up results are available for this combination technique. However, as the Spongioflex implant is also a biological implant, it can be assumed that the results will be similar to those of the CMI implant. This is also confirmed by the short interviews I conducted and a histological examination. I have used a total of 21 Spongioflex implants since November 2022. There have been 2 complications caused by the patients themselves (pull-out due to a fall or incorrect loading). One implant could be implanted again. The second implant was removed and histologically examined. This showed cell migration of cartilage cells after just 4 weeks, which supports the assumption I made above.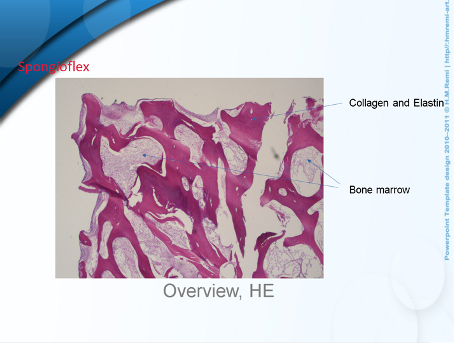
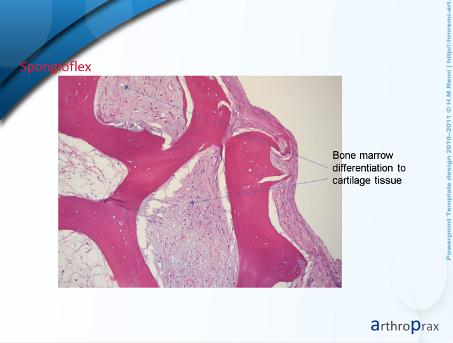
The histological section shows cartilage cell integration into the bone support tissue. An immunohistological examination is still pending.
No further complications have occurred.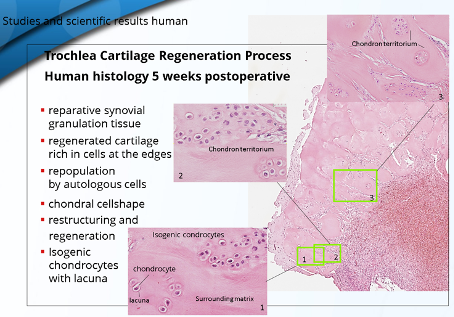
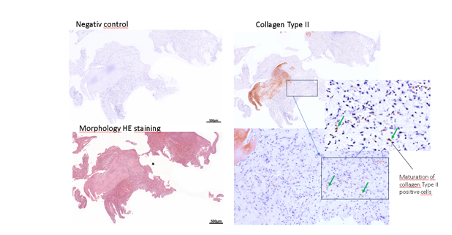
The histological examination of a defect sample of the Chondrofiller Liquid, also after 4 weeks, showed the migration of chondrocytes and the presence of collagen in the immunohistological examination.
From 12.2018 to 05.2023, a total of 23 men and 8 women were treated with this combination technique of cartilage induction and partial mensicus implantation. These 31 patients were treated with CMI in 17 cases and with Actifit in 15 cases. One patient was treated bilaterally with CMI at 7-month intervals.
All patients had either medial or lateral meniscal damage. Simultaneous treatment of medial and lateral meniscus lesions was not performed.
The follow-up examination was performed in 11 men and 5 women with an average age of 58 years (42-69 years) using the IKDC questionnaire after an average of 24 months (3-39.5 months).
It was found that one patient treated with CMI was fitted with a full or sled prosthesis after 8 months and two patients treated with Actifit after 8 and 10 months respectively.
In the CMI group, there was an average improvement from 35.1 (25.3-37.9) to 82.3 (55.8-93.1) after IKDC and with Actifit from30.4 (21.8-54) to 73.4 (56.3-83.5).
Complications (meniscal dislocation, allergic reactions, meniscal anchor dislocations) did not occur during the procedures. All patients underwent a standardised post-operative treatment regimen.
These results show that a combination of both biological procedures, with the correct indication, can avoid prosthesis implantation or, due to the lack of long-term studies, at least delay it.
Bibliography
Literature Cartilage regeneration
1. Beck OT: Cartilage repair with cell-free collagen (Chondrofiller liquid).
Clinical and MRI study.
OUP 2018; 7: 620–625 DOI 10.3238/oup.2018.0620–0625
2. Bentley, G, Biant, LC, Carrington, RWet al: A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee.
J Bone Joint Surg Br. 2003; 85: 223–30
3. Breil-Wirth A, von Engelhardt LV, Lobner, S, Jerosch J: Retrospective study of cell-free collagen matrix for cartilage repair
OUP 2016; 9: 515–20
4. Dozin B, Malpeli M, Cancedda R et al:
Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005; 15: 220–26
5. Filova E, Ramoichová M, Handl M et al.: Composite hyaluronate-type I collage-fibrin scaffold therapy of osteochondral defects in miniature pigs;
Physiol Res 2007; 56 (Suppl.1): 5–16
6. Gavenis K, Schmidt-Rohlfing B, Andereya S et al: A cellfree collagen type I device for the treatment of focalcartilage defects. Artif Organs 2010;
34: 79–83
7. Gobbi A, Karnatzikos G, Kumar A: Long-term results after microfracture treatment for full thickness knee chondral lesions in athletes;
Knee Surg Sports Traumatol Arthrosc. 2014; 22: 1986–96
8. Gotterbarm T, Breusch SJ, Schneider U, Jung M: The minipig model for experimental chondral and osteochondral defect repair in tissue engeneering: retrospectiveanalysis of 180 defects.
Lab Anim. 2008; 42: 71–82
9. Gudas R, Simonaityte R, Cekanauskas E et al.: A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children.
J Pediatr Orthop. 2009; 29: 741–8
10. Gudas R, Stankevicius E, Monastyreckiene E et al.: Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes.
Knee Surg Sports Traumatol Arthrosc. 2006; 14: 834–42
11. Hangody L, Dobos J, Baló E et al.: Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;
38: 1125–33
12. Hangody L, Füles P: Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weightbearing joints: ten years of experimental and clinical experience.
J Bone Joint Surg Am. 2003; 85-A Suppl 2: 25–32
13. Horas U, Pelinkovic D, Herr G et al: Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial.
J Bone Joint Surg Am. 2003; 85A: 185–92
14. Jung M, Tuischer JS, Sergei C et al.: Local applicationof a collagen type I/hyaluronate matrix growth and differentiation factor 5 influences the closure of osteochondral defects in a minipig model by enchondral ossification.
Growth Factors 2006; 24: 225–32
15. Schüttler KF, Schenker H, Theisen C et al.: Use of cell-free collagen type I matrix implants for the treatment of small cartilage defects in the knee: clinical and magnetic resonance imaging evaluation.
Knee Surg Sports Traumatol Arthrosc. 2014; 22: 1270–6
16. Knutsen G, Engebretsen L, Ludvigsen TC et al.: Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial.
J Bone Joint Surg Am 2004; 86-A: 455–64
17. Matzek j, Gnatowski M, Porthos Salas A, O´Donnell JM, Domzalski M, Radzimowski J: Arthroscopic utilization of Chondrodiller Liquid gel for the treatment of hip articular cartilage defects: a cohort study with 12- to 60- month follow up.
J Hip Preserv Surg 2021; 8(1):22-27
18. National Institute for Health and Care Excellence (NICE): Mosaicplasty for knee cartilage defects.
Published March 2018; (https://www.nice.org.uk/guidance/ipg607)
19. Niemeyer P, Pestka JM, Kreuz PC et al.: Characteristic Complications After Autologous Chondrocyte Implantation for Cartilage Defects of the Knee Joint.
Am J Sports Med. 2008; 36: 2091–9
20. Schneider U: Controlled, randomized multicenter study to compare compatibility and safety of ChondroFiller Liquid (cell-free 2-component collagengel) with microfracturing of patients with focal cartilage defects of the knee joint;
Video J Orthop Surg 2016; 1: 1–8
21. Schneider U, Rackwitz L, Andereya S et al.: A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee.
Am J Sports Med. 2011; 39: 2558–65
22. Steadman JR, Briggs KK, Rodrigo JJ et al.: Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up.
Arthroscopy 2003; 19: 477–484
Literature Meniscus implants
1. Bulgheroni E., Grassi A., Campagnolo M., et al. Comparative study of collagen versus Synthetic-Based meniscal scaffolds in treating meniscal deficiency in young active population.
Cartilage. 2016; 7: 29-38 (https://doi.org/10.1177/1947603515600219)
2. de Caro F., Perdisa F., Dhollander A., et al. Meniscus scaffolds for partial meniscus defects.
Clin Sports Med. 2020; 39: 83-92 (https://doi.org/10.1016/j.csm.2019.08.011)
3. Dhollander A., Condello V., Madonna V. Meniscal Augmentation and Replacement (Menaflex, Actifit, and NUsurface).
in: Farr J. Gomoll A. Cartilage Restoration. Springer, Cham2018 (ISBN: 978-3-319-77152-6)
4. Faivre B., Bouyarmane H., Lonjon G., et al. Actifit® scaffold implantation: influence of preoperative meniscal extrusion on morphological and clinical outcomes.
Orthop Traumatol Surg Res. 2015; 101: 703-708 (https://doi.org/10.1016/j.otsr.2015.06.016)
5. Filardo G., Andriolo L., Kon E., et al. Meniscal scaffolds: results and indications. A systematic literature review.
Int Orthop. 2015; 39: 35-46 (https://doi.org/10.1007/s00264-014-2415-x)
6. Grassi Alberto et al. Minimum 10-Year Clinical Outcome of Lateral Collagen Meniscal Implants for the Replacement of Partial Lateral Meniscal Defects: Further Results from a Prospective Multicenter Study.
Orthop J Sports Med 2021 May 25; 9(5):2325967121994919. doi: 10.1177/2325967121994919. eCollection 2021 May.
7. Holsten D, Andreß B: Meniscal replacement in athletes.
OUP 2014; 6: 286–291 DOI 10.3238/oup.2014.0286–0291
8. Kohli, S., Schwenck, J. & Barlow, I. Failure rates and clinical outcomes of synthetic meniscal implants following partial meniscectomy: a systematic review.
Knee Surg & Relat Res 34, 27 (2022). (https://doi.org/10.1186/s43019-022-00155-1)
9. Kovacs B.K., Huegli R., Harder D.,et al. MR variability of collagen meniscal implant remodelling in patients with good clinical outcome.
Knee Surg Sports Traumatol Arthrosc. 2021; 29: 90-99 (https://doi.org/10.1007/s00167-019-05715-9)
10. Kwon H., Brown W.E., Lee C.A., et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair.
Nat Rev Rheumatol. 2019; 15: 550-570 (https://doi.org/10.1038/s41584-019-0255-1)
11. Linke, R.D., Ulmer, M. & Imhoff, A.B. Replacement of the Meniscus with a Collagen Implant (CMI).
Orthop Traumatol 18, 453–462 (2006). (https://doi.org/10.1007/s00064-006-1188-9)
12. Leroy A, Beaufils P, Faivre B , Steltzlen C, Boisrenoult P, Pujol N
Actifit® polyurethane meniscal scaffold: MRI and functional outcomes after a minimum follow-up of 5 years Orthopaedics & Traumatology: Surgery & Research 103 (2017) 609–614.
14. Pereira H., Cengiz I.F., Vilela C., da Silva Morais A., Oliveira J.M. ,Reis R.L. et al. Emerging Concepts in Treating Cartilage, Osteochondral Defects, and Osteoarthritis of the Knee and Ankle.
Adv Exp Med Biol. 2018; 1059: 25-62 (https://doi.org/10.1007/978-3-319-76735-2_2)
15. Rodkey William G, Steadman J.Richard
Paper #30 Collagen Meniscus Implants (CMI): multicenter clinical trial results and long term follow-up
Abstract| Volume 19, ISSUE 6, SUPPLEMENT , 16-17, July 2003; (https://doi.org/10.1016/S0749-8063(03)00434-1)
16. Schenk L., Bethge L., Hirschmann A., et al. Ongoing MRI remodeling 3–7 years after collagen meniscus implantation in stable knees.
Knee Surg Sports Traumatol Arthrosc. 2020; 28: 1099-1104 (https://doi.org/10.1007/s00167-019-05714-w)
17. Schüttler K.F., Pöttgen S., Getgood A., et al. Improvement in outcomes after implantation of a novel polyurethane meniscal scaffold for the treatment of medial meniscus deficiency.
Knee Surg Sports Traumatol Arthrosc. 2015; 23: 1929-1935 (https://doi.org/10.1007/s00167-014-2977-6)
18. Shin Y.-S., Lee H.-N., Sim H.-B., et al. Polyurethane meniscal scaffolds lead to better clinical outcomes but worse articular cartilage status and greater absolute meniscal extrusion.
Knee Surg Sports Traumatol Arthrosc. 2018; 26: 2227-2238 (https://doi.org/10.1007/s00167-017-4650-3)
19. Toanen C., Dhollander A., Bulgheroni P.,et al. Polyurethane meniscal scaffold for the treatment of partial meniscal deficiency: 5-year follow-up outcomes: a European multicentric study.
Am J Sports Med. 2020; 48: 1347-1355 (https://doi.org/10.1177/0363546520913528)
20. Zaffagnini S., Grassi A., Marcheggiani Muccioli G.M., et al. MRI evaluation of a collagen meniscus implant: a systematic review.
Knee Surg Sports Traumatol Arthrosc. 2015; 23: 3228-3237m (https://doi.org/10.1007/s00167-014-3155-6)
Download the PDF version
Meniscus Implantation and Cartilage Induction

